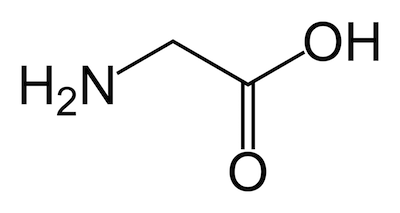
Since this isn’t in the textbook, today we’ll get some practice reading the skeletal structures of molecules.
Here are the basic rules we need to make sense of how chemists and biologists abbreviate molecular structures:
- Every place where lines (which represent covalent bonds) meet, there’s a carbon.
- Every place where lines end, there a carbon.
- And those carbons have the appropriate number of hydrogens bonded to them to satisfy the Octet Rule for carbon.
Try using these rules to draw out the full molecular structure of glycine (an amino acid, a kind of molecule we’ll talk about in the next part of the course) from its skeletal structure shown above. Then check your structure with the ball-and-stick depiction of glycine shown below.